
V3 MicroSquirt® - QuickStart Guide
Tuning and Emissions
MegaSquirt® and MicroSquirt® controllers are experimental devices intended for educational and research purposes. MegaSquirt® and MicroSquirt® controllers are not for sale or use on pollution controlled vehicles. Check the laws that apply in your locality to determine if using a MicroSquirt® controller is legal for your application.
Automotive emissions occur in several forms:
- Tail-pipe emissions: This is what most people think of as 'vehicle emissions'; the products of burning fuel in the vehicle's engine, emitted from the vehicle's exhaust system. The major pollutants include:
- Unburned Hydrocarbons (HC): Unburned hydrocarbon emissions result when fuel molecules in the engine do not burn or burn only partially. Often this is the result of ignition misfires, low compression, oil leakage past the rings or valves, or excessively rich mixtures (or conversely, lean mixtures that are too lean to ignite, resulting in a lean misfire - and *all* of the fuel passes through as unburned HC!). Hydrocarbons, once released into the atmosphere, react in the presence of nitrogen oxides and sunlight to form ground-level ozone (O3), a major component of smog (see below for details).
- Nitrogen oxides (NOx): Nitrogen oxides (technically 'mono-nitrogen oxides': NO and NO2) are generated when normally inert nitrogen in the air reacts with oxygen under the high temperature and pressure conditions inside the engine. These are exacerbated by anything that raises the combustion temperature and pressure or the availability of free oxygen: higher compression ratios, lean mixtures, retarded ignition timing, raised boost, etc. Nitrogen oxides (NOx), like hydrocarbons, are precursors to the formation of ozone (O3). They also break down in the atmosphere to form nitric acid (HNO3) which contributes to the formation of acid rain.
- Carbon monoxide (CO): Carbon monoxide is a product of incomplete combustion and occurs when carbon in the fuel is partially oxidized rather than fully oxidized to carbon dioxide (CO2). It is most likely to occur with excessively rich mixtures. Carbon monoxide reduces the flow of oxygen in the bloodstream and as a result is particularly dangerous to people with heart disease.
- Carbon dioxide (CO2): Emissions of carbon dioxide are an increasing concern as its role in global warming as a greenhouse gas has become more apparent in recent years.
- Evaporative emissions: These are produced by the evaporation of raw fuel, and can be a significant contributor to urban smog, since the heavier hydrocarbon molecules stay close to ground level rather than dissipating into the upper atmosphere. Fuel can evaporate from a vehicle in a number of ways:
- Gas tank venting: as the temperature rises from cooler overnight temperatures to the heat of mid-day (plus any additional radiant heat from the exhaust system, etc.) the gasoline's vapor pressure increases as well, increasing the pressure inside the fuel tank by several pounds per square inch above atmospheric pressure. A some point this pressure must be relieved, and before emissions control it was simply vented into the atmosphere as unburned hydrocarbon emissions (HC). Now, however, this function is often under the control of the ECU, and is carefully managed. Such gases may be trapped in charcoal canister and fed to the engine's intake system at appropriate times, for example.
- Running losses: the escape of gasoline vapors (unburned hydrocarbons) from the hot engine, such as when the engine is shut off, and residual fuel remaining in the intake tract evaporates and can escape into the atmosphere if it isn't carefully planned and managed.
- Re-fuelling losses: can create a lot of hydrocarbon vapor emission (HC). The empty space inside a vehicle's tank is filled with hydrocarbon gases, and as the tank is filled, these gases are forced out into the atmosphere. In addition, there is loss from further evaporation as the fuel tank is opened for re-fuelling, and fuel spillage.
- Life cycle emissions: These are produced as a direct result of the activities associated with the manufacture, maintenance, and disposal of the automobile. Life cycle emissions include such items as:
- Manufacturing power supply requirements mean that there may be emissions from the power generating source (coal fired plant, etc.). These include the power requires to run plants, as well as the power required to smelt the metals used, etc.
- Volatile solvents used a number of stages in the manufacturing process (painting, plastics manufacturing, cleaning, etc.).
- Out-gassing of synthetic materials utilized to reduce weight and simplify manufacturing (such as 'lost foam' casting processes, etc.).
- Maintenance requirements such as oil and filter changes, battery replacement, repainting when necessary (including collision damage), etc.
- Disposal requirements including contaminated lubricants, air conditioning refrigerant, tires, heavy or rare-earth metals, and landfill space requirements.
Traditionally, gasoline is a complex mixture of hydrocarbons (molecules made up of hydrogen atoms on a chain of carbon atoms) of the form CαHβ. It might have trace amounts of nitrogen as well. Oxygen was not historically a part of the structure (though is often included now, more on this below!). The average ratio of carbon to hydrogen in the gasoline hydrocarbon mix is around 8 carbon atoms to 18 hydrogen atoms, i.e. C8H18. By the way, C8H18 is the formula for 'octane' ('oct' implies '8'), and is the basis of the 'octane rating' for knock.
A simplified chemical equation for perfect gasoline/air combustion (the ratio of fuel to air required for perfect combustion is known as stoichiometric (pronounced 'stoy-eék-ee-o-metric') is:
C8H18 + 12.5 O2 → 8 CO2 + 9 H2O
The oxygen (O2) is consumed from the intake air. Nitrogen (N2) is also present in the atmospheric air, but ideally does not participate in any reactions (it is quite inert at low temperatures). Note that the combustion products are carbon dioxide (CO2) and water (H2O), if the combustion is 'perfect'.
However, in the real world, not all the fuel burns:
- some fuel is hidden in the relatively cool and shielded crevice volumes of the combustion chamber such as just above the top ring,
- some portion of the fuel nearest the relatively cool combustion chamber surfaces are 'quenched' before burning, and
- some of the gasoline does not have oxygen available during the very short combustion period for a number of reasons - especially if the mixture is rich.
The result is unburned hydrocarbon (HC) emissions (which can also refer to hydrocarbons that are only partially burned).
As well, the heat of combustion may cause the normally inert nitrogen (N2) that composes ~80% of the intake air to break down and combine with oxygen, resulting in oxides of nitrogen emissions (NOx) - single nitrogen atoms with one or more oxygen atoms attached. (Note this is different from nitrous oxide, which is N2O, i.e. two nitrogen atoms are attached to one oxygen atom.) NOx is especially likely to be produced if the combustion temperature is high (high compression, etc.) and if the mixture is lean (because there is excess oxygen remaining even after burning all the fuel).
Finally, some of the fuel won't be able to combine completely with oxygen (which may be locally consumed within the combustion chamber - especially if the mixture is rich), and the result is carbon monoxide (CO) emissions.
So the 'real world' chemical equation for gasoline combustion looks more like:
C8H18 + ~12.5 O2 + ? N2 → <8 CO2 + <9 H2O + ? HC + ? NOx + ? CO + ? H2
The numbers on the right hand side (the products of combustion) vary considerably with operating conditions, a multitude of design factors, and fueling/ignition strategies.
There is a general relationship between the air/fuel ratio and the regulated tail pipe emissions put out by a given engine design:
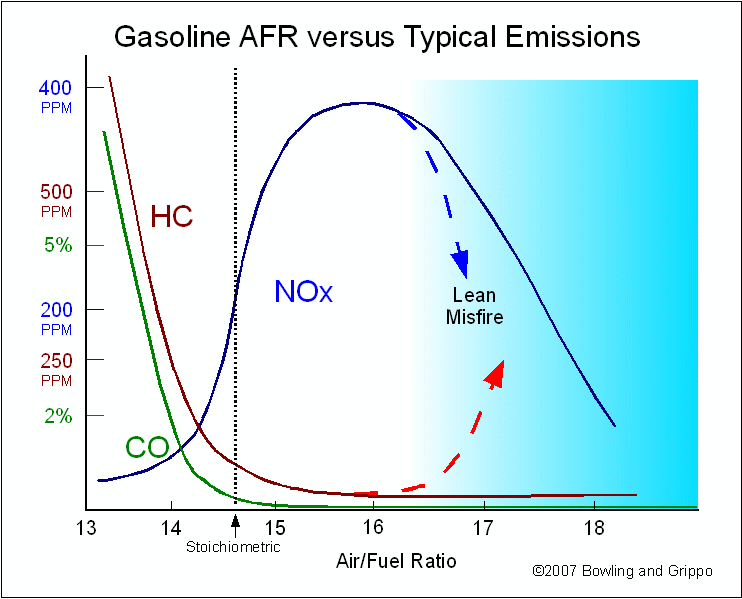
What is Smog?
Smog (a term originating in England from the days when coal and peat fired heating created a thick mixture of smoke and fog - "smog"!) is an accumulation of ozone and volatile organic compounds (called VOC) such as peroxyacetyl nitrate (PAN). These are referred to as 'secondary pollutants', because they are formed by the reaction of precursor pollutants emitted by burning fossil fuels, such as nitrogen oxides and hydrocarbons. The energy from sunlight makes the smog forming reactions possible. The result is photochemical smog, the brown haze in the sky that is especially prevalent on hot sunny days.
To form photochemical smog, three main ingredients are needed: nitrogen oxides (NOx), hydrocarbons (HC), and energy from the sun in the form of ultraviolet light (which we will denote as hν = E - the energy of a photon).
In the atmosphere, the nitric oxide resulting from gasoline combustion combines with atmospheric molecular oxygen to form nitrogen dioxide (NO2) within a few hours.
2 NO + O2 → 2 NO2
Nitrogen dioxide (NO2) absorbs sunlight energy and splits to form nitric oxide and atomic oxygen:
NO2 + hν → NO + O
Then, in sunlight, the atomic oxygen (O) combines with molecular oxygen gas (O2) to form ozone (O3):
O + O2 + hν → O3
If there were no other precursors present, ozone and nitric oxide would then react to form nitrogen dioxide and molecular oxygen:
O3 + NO ←hν→ NO2 + O2
This last reaction can proceed in either direction, depending on temperature and the amount of sunlight. If there is a lot of sunlight and the temperature is high, the equation moves to produce the products on the left, and more ozone is produced.
However, engines also emit hydrocarbons (HC) as well as oxides of nitrogen. Hydrocarbons (HC) are the other main precursor forming photochemical smog (besides NOx). When hydrocarbons are present, nitric oxide (NO) preferentially reacts with them instead of reacting with the ozone. This reaction can produce a variety of toxic products, such as a volatile compound (VOC) known as PAN (peroxyacetyl nitrate), CH3COOONO2. (Organic compounds are all chemical compounds containing carbon-hydrogen (C-H) bonds of covalent character. The U.S. Environmental Protection Agency defines a VOC as any organic compound that participates in a photoreaction.) The reactions of NOX and unburned hydrocarbons (HC) are:
NO + HC → CH3COOONO2 and various other volatile organic compounds
NO2 + HC → CH3COOONO2 and various other volatile organic compounds
The result is that there are two problems created by the reaction of the tail pipe pollutants in the atmosphere:
- Considerable quantities of volatile reactive organic compounds, like PAN, are generated directly that result in the smog you see and the health problems it creates, and
- When the nitric oxide (NO) reacts with hydrocarbons, it is not available to react with ozone to break it back down to molecular oxygen. As a direct result, ozone may accumulate to damaging levels.
The effects resulting from exposure to photochemical smog range from irritations of the respiratory tract (such as coughing and wheezing and breathing discomfort) and small biochemical or physiological changes, to severe breathing difficulties, markedly reduced lung capacity and efficiency, and aggravation of existing respiratory and cardiovascular conditions.
Ozone affects lung functioning in a variety of ways, causing inflammation in the respiratory tract and damage to lung tissue, contributing to reduced inhalation capacity and lung function.
Studies show there is no safe level of smog. Damaging health effects from exposure to smog can be caused by exposure to large concentrations over a short time span, or by chronic exposure to small amounts over long periods of time.
In addition to the effect on human health, ozone can kill plant cells, reduce plant growth, and make plants more susceptible to damage from other causes. Oxidants such as ozone can also corrode and degrade many common materials such as rubber, nylon, fabric, and paint.
Acid Rain
In addition to smog, automotive emissions can contribute to acid rain. In the air, nitric oxide (NO) is oxidized to nitrogen dioxide (NO2), which in turn reacts with water in the air to give nitric acid (HNO3). This acid dissociates in water to yield hydrogen ions and nitrate ions (NO3-) - i.e., acid rain!
3 NO2 + H2O → 2 HNO3 + NO
Acid rain triggers a number of inorganic and biochemical reactions with harmful effects on the environment:
- Many lakes have become so acidic that fish and other creatures cannot live in them,
- Degradation of soil minerals by acid rain produces metal ions that are then washed away in the runoff:
- Toxic ions, such as Al3+, are released into the water supply, harming everything in the water and all life that depends on the water (for drinking, etc.).
- Important minerals, such as Ca2+, are leached from the soil, which can deprive trees and agricultural crops of essential minerals needed for life and healthy growth.
- Atmospheric acidity is transported over long distances by wind currents, so acid-rain effects can be have effects far from where pollutants was originally generated.
|
A catalytic converter can further reduce tail pipe emissions substantially below what the engine itself produces. This eliminates them from being able to become the precursors to photochemical smog in the atmosphere. A 3-way catalytic converter will support the following chemical reactions in the exhaust gas stream:
The net result is that NOx, CO, and HC are reduced (while CO2 and H2O are increased somewhat).
However, the catalytic converter requires a near stoichiometric mixture to for maximum efficiency, because it only as a limited ability to store oxygen. If the mixture is too rich, there won't be enough oxygen to oxidize the unburned hydrocarbon and carbon monoxide. If the mixture is too lean, the oxides of nitrogen won't be completely reduced. ('Oxidation' is a chemistry term meaning to add oxygen to a molecule, 'reduction' is a chemistry term mean to remove oxygen from a molecule)
The catalytic converter must also reach its 'light off' temperature (around 400° to 600°F) before it will catalyze any of the above reactions - so getting it hot quickly is very important to reducing 'start-up' emissions. This often means a bit of a trade off - for example, retarded timing and a richer mixture to maximize the heat in the exhaust gases while warming up. (Manufacturers have be searching for a method to store converter heat on shut down (the operating temperature may reach 1200° to 1600°F) so it can be used to heat the converter on start up - a number of methods have been proposed, but none have been implemented in production vehicles at the time this is written, to our knowledge.)
The narrow band oxygen sensors are very sensitive to the exhaust gases that result from a stoichiometric mixture. Richer than stoichiometric (by as little as 0.2:1 AFR!) and they give a signal near 1 Volt. Leaner than stoich. and they give a signal near 0 volts. The result is that the narrow band sensor makes a good 'switch' to tell the fuel system to add or subtract fuel to meet the requirements of the catalytic converter for minimal emissions.
For research and educational purposes, properly tuning your engine for minimal emissions using a MegaSquirt® EFI controller generally requires expensive test equipment. However, as a rough initial plan for such a research program, you can:
- Make sure your ignition system is in perfect working condition, as well as any normal emissions related maintenance items. A tune-up (distributor cap, rotor, wires, spark plugs, PCV, EGR, etc.) is a good idea before emissions testing. Ignition misfires will greatly increase the unburned hydrocarbon (HC) emissions, while reducing NOx and CO emissions (and ruining fuel economy),
- Make sure your engine is equipped with a functional 3-way catalytic converter(s) if possible,
- Set your EGO Switch Point to 0.450 volts for a narrow band sensor, stoichiometric for a wide band sensor (2.500 volts for the DIY-WBO2 for example). This provides for real-time stoichiometric mixture control for a chemically 'perfect' mixture. Note that for emissions control purposes, a narrow band sensor may be a better choice than a wide band sensor. This is because the narrow band sensor is not affected by calibration issues, it not affected as much by changing exhaust gas pressure and temperature, and has a much stepper response curve near the switch point.
- Retard your ignition timing by somewhat - a few degrees (this creates a hotter exhaust gas mixture that prolongs the burn and also helps the catalytic converters to operate at peak efficiency). It will increase NOx somewhat, however,
- Increase your idle speed slightly - if the engine idles well at 750 rpm, but stumbles a bit if set lower, set it to about 850-900 rpm, this increases the mixture speed, and thus the uniformity and vaporization,
- If you have an adjustable electric fan, increase the fan 'ON' temperature to about 200°F to 215°F. The increased temperature increases cylinder to cylinder mixture uniformity, promotes vaporization of the fuel for a more complete burn, and can reduce friction somewhat,
- If you have an oiled 'lifetime' air filter, replace it with a plain paper filter for testing (the oil in the filter might be drawn into the intake air mixture, and can drive up HC emissions),
- Make sure your vehicle is fully warmed-up before testing. Cold engines require rich mixtures (because the gasoline is slower to vaporize when cold), and combustion efficiency is decreased by the cool combustion chamber surfaces. As well, neither the oxygen sensor nor the catalytic converter work until they are warmed up. The O2 sensor will often have a built in heating element (that will normally heat the sensor to operating condition in 30 seconds or less), but the catalytic converter does not have a heating element and relies on hot exhaust gases to warm it enough make it functional.
- Add some alcohol (either oxygenated blends of gasoline, or a separate source of methanol/ethanol) to your fuel tank. This helps by introducing additional oxygen into the combustion process. Gasoline has no oxygen atoms in its composition - remember it is a hydrocarbon.
Alcohols have one oxygen atom, and are of the form CαHβOH (where typically β = 2α+1). For example, ethanol: C2H5OH, methanol: CH3OH. The additional oxygen helps ensure a more complete burn of the gasoline, and lowered CO emissions. For testing the emissions response to ethanol or methanol, you might start with 5% added ethanol or methanol (However note that adding ethanol, and especially methanol, has the effect of 'leaning out' the mixture. The more you add, the more you will effectively lean the mixture).
Gasoline distributors are sometimes required by the EPA to blend certain amounts of alcohol (ethanol) into gasoline in winter months in areas that have high carbon monoxide emissions. These mixes are generally 5% to 10% ethanol and the rest gasoline, and are called 'oxygenated blends'. In some regions, gasoline has some ethanol in it all year round. There is more on ethanol blends here: www.megamanual.com/flexfuel.htm
Here is an example of how a few changes affected one engine:
|
Baseline |
Timing
Retarded 10° |
Timing
Advanced 10° |
Mixture
richened |
Mixture
leaned |
| HC |
1.00 |
0.78 |
1.14 |
1.39 |
0.88 |
| CO |
14.5 |
11.6 |
13.0 |
81.0 |
9.8 |
| NOx |
2.91 |
1.89 |
5.18 |
0.71 |
3.03 |
O2
Not usually tested |
22.3 |
19.8 |
23.8 |
17.4 |
25.4 |
CO2
Not usually tested |
305 |
307 |
298 |
246 |
310 |
You can see that retarding the timing and leaning the mixture slightly gives the best possible compromise, increasing only the NOx slightly among the regulated emissions. (The above was without a catalytic converter. With a converter, emissions would have been best at a stoichiometric mixture and the timing retarded somewhat.)
If you have any questions or problems that can't be answered from the links above, or a search the MicroSquirt® manual:
you can ask questions at the MicroSquirt® support forum which is at: www.microsquirt.com Click the links for more information.
MegaSquirt® and MicroSquirt® controllers are experimental devices intended for educational purposes.
MegaSquirt® and MicroSquirt® controllers are not for sale or use on pollution controlled vehicles. Check the laws that apply in your locality to determine if using a V3 MicroSquirt® or MicroSquirt® controller is legal for your application.
©2011 Bruce Bowling and Al Grippo. All rights reserved. V3 MicroSquirt® and MicroSquirt® are registered trademarks. This document is solely for the support of V3 MicroSquirt® boards from Bowling and Grippo.




